-
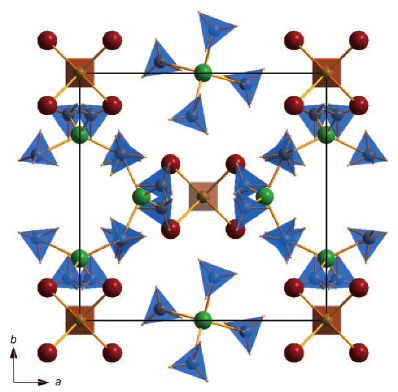
Novel sodium aluminium borohydride containing the complex anion [Al(BH4,Cl)4]−
I. Lindemann, R.D. Ferrer, L. Dunsch, R. Cerny, H. Hagemann, V. D'Anna, Y. Filinchuk, L. Schultz and O. Gutfleisch
Faraday Discussions, 151 (2011), p231-242


DOI:10.1039/C0FD00024H | unige:16758 | Abstract | Article HTML | Article PDF

The synthesis of a novel alkali-metal aluminium borohydride NaAl(BH4)xCl4−x from NaBH4 and AlCl3 using a solid state metathesis reaction is described. Structure determination was carried out using synchrotron powder diffraction data and vibrational spectroscopy. An orthorhombic structure (space group Pmn21) is formed which contains Na+ cations and complex [Al(BH4,Cl)4]−anions. Due to the high chlorine content (1 ≤ x ≤ 1.43) the hydrogen density of the borohydride is only between 2.3 and 3.5 wt.% H2 in contrast to the expected 14.6 wt.% for chlorine free NaAl(BH4)4. The decomposition of NaAl(BH4)xCl4−x is observed in the target range for desorption at about 90 °C by differential scanning calorimetry (DSC), in situ Raman spectroscopy and synchrotron powder X-ray diffraction. Thermogravimetric analysis (TG) shows extensive mass loss indicating the loss of H2 and B2H6 at about 90 °C followed by extensive weight loss in the form of chloride evaporation.